Morinaga Milk Obtains the Registration of New Food Ingredient in China for use of its Probiotic Bifidobacteriuminfantis M-63 in Infant Foods
09.08.2024 - 06:35:45This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240808893280/en/
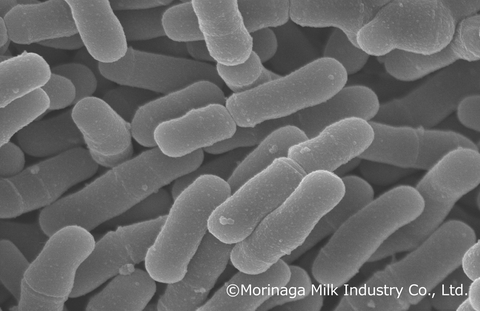
Bifidobacterium infantis M-63 is a unique probiotic strain of human-residential bifidobacteria (HRB) – bifidobacteria that naturally reside in the human gut – and is renowned for its ability to utilize human milk oligosaccharides (HMO) found in breast milk, significantly improving the intestinal environment of healthy full-term infants. (Graphic: Business Wire)
*1 Source: Public announcement published on the website of the China National Center for Food Safety Risk Assessment (August 5, 2024)
The newly registered Bifidobacterium infantis M-63 joins Morinaga Milk’s other approved strains, Bifidobacterium breve M-16V and Bifidobacterium longum BB536. All three strains are now permitted for use in foods for children under three years old in China, meeting the country's stringent nutritional and safety standards.
Expanding Global Presence
This approval aligns with Morinaga Milk’s 10-year vision to become a globally recognized company with a significant international presence. As part of this vision, the company aims to achieve a Global Business sales ratio of 15% or more by the fiscal year ending in March 2029. The approval of Bifidobacterium infantis M-63 represents a strategic step towards this goal, allowing Morinaga Milk to strengthen its bacteria business in the Chinese market, which accounts for more than 40% of the global formula milk market and is the second-largest supplement market worldwide2.
*2 Source: Survey by Euromonitor International (May 2024), based on retail sales prices
Health Benefits of Bifidobacterium infantis M-63
Bifidobacterium infantis M-63 is a unique probiotic strain of human-residential bifidobacteria (HRB) – bifidobacteria that naturally reside in the human gut – and is renowned for its ability to utilize human milk oligosaccharides (HMO) found in breast milk, significantly improving the intestinal environment of healthy full-term infants. With China's recent approval of HMO use in formula milk in October 20233, the combination of Bifidobacterium infantis M-63 and HMO is expected to support the growth of beneficial intestinal bacteria, enhancing the development and health of infants and toddlers4.
*3 Source: National Health Commission of the People’s Republic of China, No. 8, 2023 (Sept. 22, 2023)
*4 Source: Mintel, “HMOs approval in China infant formula is a game changer” (Jan. 2024)
|
Probiotics strain of bifidobacteria registered as New Food Ingredients in China |
|
|
Strain |
Registration date |
|
Bifidobacterium breve M-16V Registered name: ????? M-16V |
2016 |
|
Bifidobacterium longum subsp. longum BB536 Registered name: ???????? BB536 |
2022 |
|
Bifidobacterium longum subsp. infantis M-63 Registered name: ?????????M-63 |
2024 |
|
* All three strains are approved for use in foods for infants and toddlers under the age of three. |
|
Commitment to Quality and Research
Morinaga Milk has been at the forefront of bifidobacteria research since discovering Bifidobacterium longum BB536 in 1969. The company leads globally in the number of clinical research papers on bifidobacteria, underlining its commitment to advancing scientific understanding and improving public health.
Future Prospects
The registration of Bifidobacterium infantis M-63 paves the way for its use in a wide range of products. Morinaga Milk plans to leverage this approval to expand collaborations with major formula milk manufacturers and promote supplements and other related products in China and globally. By providing accurate information on the benefits of Bifidobacterium infantis M-63 to consumers and partners, Morinaga Milk aims to enhance the health and nutrition of people worldwide.
About Morinaga Milk Industry
Morinaga Milk Industry Co., Ltd. is one of the leading dairy product companies in Japan, with over a century of history in harnessing the nutritional properties of dairy products and functional ingredients. Morinaga Milk is also a key global probiotics manufacturer that excels in innovative technology and offers a premium line of probiotics and functional ingredients worldwide. Since the 1960s, Morinaga Milk has been engaged in research on the safety, functional health benefits, and mechanisms of action of probiotic bifidobacteria to better understand their role in maintaining human health. For more information about Morinaga Human-Residential Bifidobacteria (HRB) probiotics, please visit us at https://morinagamilk-ingredients.com/.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240808893280/en/

